The Sad Tale of an On-Sale Bar Created by a “Business Associate”
Intellectual Property News
The Federal Circuit recently reversed a lower court’s summary judgment that a medical device design patent was not invalid under the on-sale bar. As a result, the patent owner (Junker) lost the $1.25M infringement damages awarded to him against the two medical device companies he sued in the district court of the District of Pennsylvania in 2013. The Junker v. Medical Components, Inc. decision serves as a reminder to inventors to take the appropriate steps to protect an invention before moving forward to commercialize. The back story of the invention is a depressing tale of what could happen if you disclose an invention to a potential business associate that flagrantly disregards confidentiality agreements.
Background
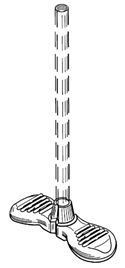 Junker’s design patent (D450,839: the ʼ839 patent) covers the “introducer sheath” shown to the right, which a component of catheter kits. The application for the ʼ839 patent was filed on February 7, 2000. When Junker sued Medical Components, Inc. and Martech Medical Products, Inc. (MedComp) for infringement in 2013, MedComp argued the design was invalid under the on-sale bar articulated in Section 102(b). Section 102(b) says that a patent is invalid if “the invention was . . . on sale in this country, more than one year prior to the date of the application for patent in the United States.” The on-sale bar is triggered if, before that critical date, the claimed invention was both: (1) the subject of a commercial offer for sale and (2) ready for patenting.
Junker’s design patent (D450,839: the ʼ839 patent) covers the “introducer sheath” shown to the right, which a component of catheter kits. The application for the ʼ839 patent was filed on February 7, 2000. When Junker sued Medical Components, Inc. and Martech Medical Products, Inc. (MedComp) for infringement in 2013, MedComp argued the design was invalid under the on-sale bar articulated in Section 102(b). Section 102(b) says that a patent is invalid if “the invention was . . . on sale in this country, more than one year prior to the date of the application for patent in the United States.” The on-sale bar is triggered if, before that critical date, the claimed invention was both: (1) the subject of a commercial offer for sale and (2) ready for patenting.
The basis for this invalidity defense stemmed from a business relationship that Junker pursued before he filed his patent application for the ʼ839 patent. More particularly, as set forth in the appellate opinion, Junker sought out James Eddings and his company Galt in an effort to find a suitable manufacturer for his sheaths. Junker and Eddings entered into a non-disclosure agreement (1998 NDA) before Junker disclosed his prototype design to Eddings. Junker subsequently provided an updated design to Eddings and Eddings, using a developer, procured final production drawings based on the Junker’s design. But, on January 8, 1999, through his newly formed company Xentek (and presumably without Junker’s knowledge), Eddings responded to a request from Boston Scientific “detailing bulk pricing information for variously sized peelable introducer sheath products” (BS Letter).
Lower Court Decision
The parties agreed that the BS Letter was sent more than one year before the February 7, 2000 filing date of the ʼ839 patent. What the parties did not agree upon was whether the BS Letter contained a commercial offer for a sale of a product embodying the design claimed in the ʼ839 patent. The district court ruled as a matter of law that the BS Letter was “a preliminary negotiation, not a definite offer,” focusing on the multiple uses of the word “quotation” and the invitation to further discuss specific requirements. At trial, the district court found that the MedComp failed to prove invalidity on its remaining defenses, but that Junker met his burden of proof on infringement and that the infringement was willful. Junker was awarded $1,247,910 in disgorged profits and MedComp appealed.
Appellate Reversal
Disagreeing with the lower court, the Federal Circuit concluded that the letter was a specific offer for sale. In particular, the appellate panel explained that the BS Letter was a direct response Boston Scientific’s request for quotation and “a specific offer to Boston Scientific to take further action.” Indeed, the BS letter included a number of terms specific to a commercial contract like prices for “shipment in bulk, non-sterile” and payment terms. Junker’s argument that the BS Letter omitted “essential terms,” e.g., the size and quantity of product being purchased, was rejected by the panel as too stringent. The panel further explained that the uses of the word “quote” in the BS Letter did not outweigh
[t]he completeness of the relevant commercial sale terms in the letter itself . . . [and] multiple offers for sale, any one of which Boston Scientific could have simply accepted to bind the parties in a contract.
The reversal of the district court’s summary judgment of no invalidity rendered the remaining issues of infringement and damages moot, and Junker lost his damages award against MedComp.
Key Takeway
Is there anything that Junker could have done differently? Hindsight is obviously 20/20, but the most conservative route that Junker could have taken—apart from avoiding Eddings altogether—was to file a patent application prior to (or at least soon thereafter) initiating manufacturing discussions with Eddings. And, even if Junker was not prepared to file an application for examination when he started talking with Eddings, he could have at least filed a provisional patent application to secure a filing date prior to disclosing his invention to Eddings.
That being said, it is important to note that you cannot file a provisional design patent application. As such, if Junker really wanted to protect only the ornamental features of his invention (which, based on the next section, seems, at a minimum, debatable), the provisional patent application would have first needed to be converted to a utility non-provisional patent application, and then the design patent application could have been filed claiming priority to the pending utility non-provisional patent application. Of course, the drawings in the earlier-filed utility non-provisional application would have needed to adequately support the drawings in the later-filed design application. While this may seem on its face like a costly route, if you consider the loss of the damages award and the attorney fees expended on litigation, the road not taken appears to be pretty affordable and certainly less frustrating.
Going Down the Rabbit Hole
If you aren’t at this point asking yourself whether Eddings acted without Junker’s consent when he sent the BS Letter, consider yourself encouraged to read the decision once again and “mind the gaps.” The appellate opinion left many unanswered questions that required further exploration of the Junker / Eddings story—at least for this author. Here is some of what the trip down the rabbit hole uncovered.
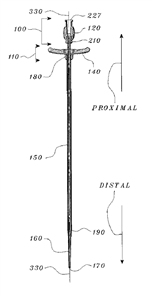 After Eddings provided Junker with the final production drawings, Eddings / Galt filed a utility patent application on January 13, 2000—naming as the inventor the developer it hired to finesse Junker’s design for the catheter introducer. Once Junker learned that Eddings had filed a patent application on the catheter introducer, Junker filed his patent application on February 7, 2000, which issued about a year and a half later on November 20, 2001 as the ʼ839 patent. A few months later, Galt’s patent application issued on January 8, 2002 as U.S. Patent No. 6,336,914 (“the ʼ914 patent”). The drawing shown to the right generally illustrates what is described and claimed in the ʼ914 patent, i.e., a dilator portion 100 and an introducer portion 110 that have a common longitudinal axis 330.
After Eddings provided Junker with the final production drawings, Eddings / Galt filed a utility patent application on January 13, 2000—naming as the inventor the developer it hired to finesse Junker’s design for the catheter introducer. Once Junker learned that Eddings had filed a patent application on the catheter introducer, Junker filed his patent application on February 7, 2000, which issued about a year and a half later on November 20, 2001 as the ʼ839 patent. A few months later, Galt’s patent application issued on January 8, 2002 as U.S. Patent No. 6,336,914 (“the ʼ914 patent”). The drawing shown to the right generally illustrates what is described and claimed in the ʼ914 patent, i.e., a dilator portion 100 and an introducer portion 110 that have a common longitudinal axis 330.
Junker then sued Eddings in the northern district of Texas for infringement of the ʼ839 patent and breach of contract based on Eddings / Galt’s violation of the 1998 NDA. In response, Galt claimed that the ʼ839 patent was invalid because “Junker was not the first to invent the claimed design.” More specifically, Galt alleged that the ʼ839 patent was invalid under 35 U.S.C. § 102(e) based on the earlier filing date of the ʼ914 patent, or alternative under §§102(f) (which bars the issuance of a patent where an applicant did not invent the subject matter being claimed and sought to be patented), and/or 102(g)(2) (which bars the issuance of a patent where another made the invention in the United States before the applicant and had not abandoned, suppressed, or concealed it). The jury did not buy Galt’s arguments and found that the ʼ839 patent was valid and infringed by Galt and awarded Junker over $800k in damages for that infringement. On appeal, the Federal Circuit left the jury’s validity and infringement verdict and damages award undisturbed.
The jury also found that Galt had breached the 1998 NDA, but found that “the breach had not caused Junker any damages.” The lower court also denied Junker’s request to impose a constructive trust on the ʼ914 patent as a remedy for Galt’s breach. And, even though Junker tried to reverse the complete lack of remedy for the breach on appeal, the Federal Circuit concluded that a) because “[t]he proper measure of recovery for a breach of contract claim is the loss or damage actually sustained,” the jury’s decision to not award Junker compensatory damages was proper in lack of the speculative nature of the damages and b) Junker did not meet the requirements to have a constructive trust placed on the ʼ914 patent.
Even though it is legally understandable, the fact pattern remains hard to digest. To bring this full circle, Junker was awarded no damages from the breach of the 1998 NDA because they were merely speculative, but the breach not only resulted in a patent awarded to Galt[1], it also enabled the offer for sale that ultimately killed the ʼ839 patent and prevented Junker from obtaining any damages against MedComp. Ouch.
[1] It is worthwhile to note that during the litigation against MedComp, MedComp proffered an affirmative defense that alleges a vastly different side of the story. In fact, MedComp alleged that Junker stole Galt’s developer’s drawings and filed them with the USPTO as if they were his own.

